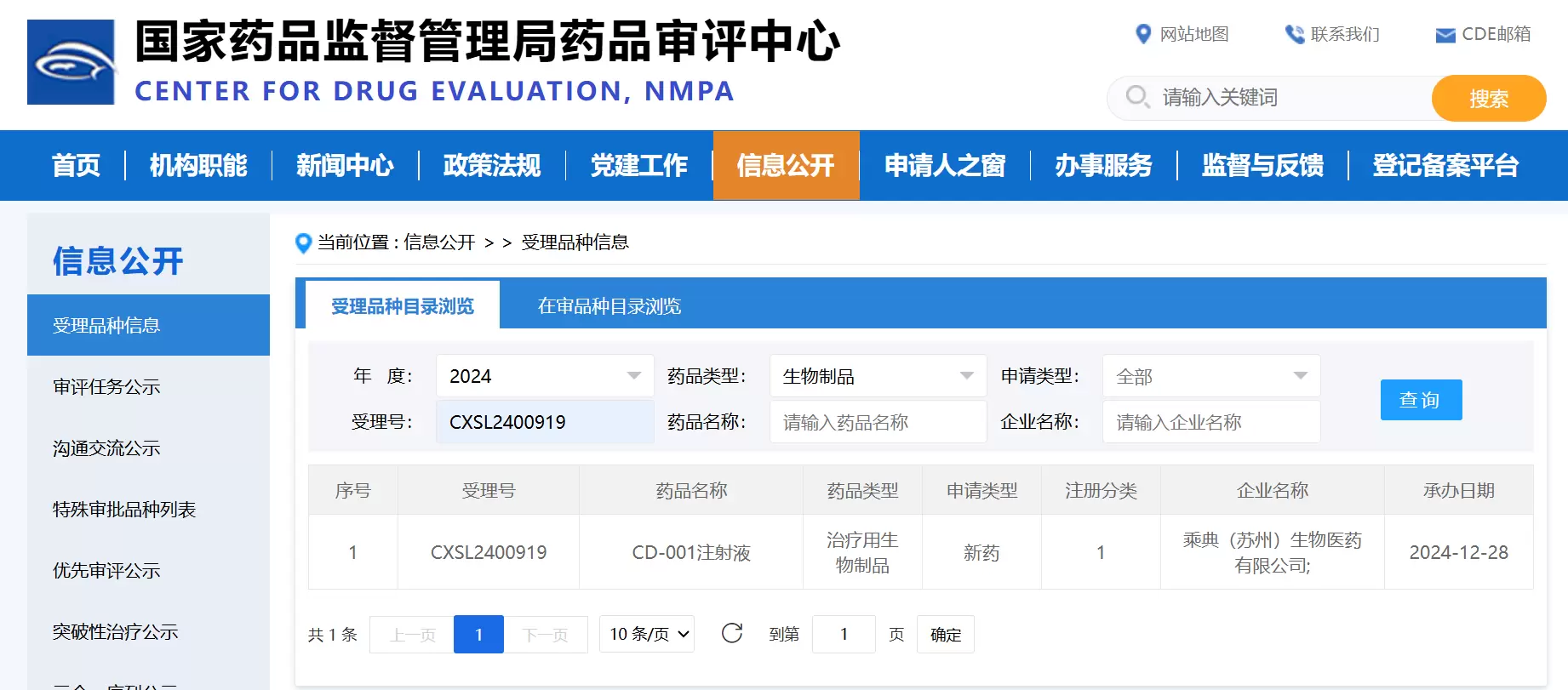
SUZHOU, China, Dec. 30, 2024 - CD (Suzhou) Biopharma announced that following the previous IND clearance from the U.S. Food and Drug Administration (FDA) and China's National Medical Products Administration (NMPA) for advanced solid tumors, its lead program CD-001 has received IND application acceptance from the Center for Drug Evaluation (CDE) of NMPA for a new indication in advanced hematological malignancies, with the acceptance number CXSL2400919. This domestic IND acceptance for the new indication demonstrates the broad therapeutic potential of this drug candidate in oncology and marks its entry into an accelerated clinical development phase targeting multiple indications.
About CD-001
CD-001, the company's leading clinical candidate, is built on its proprietary Bispecific Fusion Protein (BsFP) platform. This potential therapy, designed to target PD-1 positive CD8+ T cells using an anti-PD-1 antibody and engineered IL-21 mutant, aims to address unmet medical needs in oncology and viral infections.
About CD (Suzhou) Biopharma
Founded in 2021, CD Biopharma is a rapidly advancing clinical-stage biotech company focused on developing innovative therapies across a broad spectrum of immunotherapy areas, including oncology, viral infections, and autoimmune diseases. The company's proprietary Bispecific Fusion Protein (BsFP) platform and IMmune-Enhanced (IME) cell technology, which enable advanced cell modification, continue to drive breakthrough research and clinical outcomes that transform patient care. CD Biopharma is headquartered in Suzhou, China, with additional research operations in Beijing, China.

